Introduction
Have you explored titanium properties? They are truly impressive! Titanium, element 22, is a strong, lightweight metal with unique traits. It holds a vital place in industry and medicine today. As of May 2025, research on titanium continues to grow. Therefore, this article examines titanium properties in detail. We will review its discovery, applications, and chemical nature. Are you ready to learn? Let’s begin our exploration!
Titanium strengthens products across various sectors. Its abundance yet difficulty to process intrigues experts. How does it shape modern life? Its durability fascinates engineers. Moreover, its potential in new fields remains vast. Titanium properties provide key insights for innovation. For instance, its use in aerospace is well-known. Additionally, it supports sustainable manufacturing. Its resistance to harsh conditions stands out. It also promotes green technology solutions. Its role in reducing industrial waste is notable. It enhances construction materials for durability. Why is it so valuable? Let’s investigate further together.
What Are the Characteristics of Titanium?
Titanium properties define a remarkable metal. It has a silvery color, strong yet lightweight build. Its atomic weight is 47.87, with a melting point of 1,668°C. Titanium shows high strength-to-weight ratio, beating steel. This makes it ideal for structural uses. Also, it resists corrosion in seawater and acids. Its boiling point reaches 3,287°C, indicating great stability. Titanium properties shine under extreme heat. It resists flame damage effectively. It withstands radiation in harsh environments.
Titanium properties include excellent thermal resistance. Its crystal structure is hexagonal close-packed. When alloyed, it gains even greater toughness. For example, titanium alloys are common in aircraft. Its presence in Earth’s crust—about 0.63%—is notable. Furthermore, it reflects light with a subtle luster. Its flexibility allows shaping into thin sheets. It also withstands high mechanical stress. These traits make it ideal for extreme conditions. Its resistance to fire enhances safety. Thus, titanium properties distinguish it from other metals. Let’s explore its origin next.
How Was Titanium Discovery Made?
Titanium discovery marks an important milestone. In 1791, William Gregor first identified it in Cornwall. He found it in a mineral called ilmenite. In 1795, Martin Heinrich Klaproth confirmed it. He named it “titanium” after the Titans of Greek myth. The name reflects its strength. Early extraction was challenging due to its reactivity. Initial samples were impure and hard to isolate. Early tests required careful handling. Initial analyses involved complex procedures.
The discovery used chemical analysis methods. Titanium’s white compounds were a key hint. Initially, it was detected in rutile and ilmenite ores. Titanium discovery enriched metallurgical science. Later, advanced techniques improved its purity. Its identification required persistent experimentation. Chemists overcame obstacles over decades. The process refined over time with new tools. Early efforts laid the foundation for its use. Therefore, it paved the way for modern metallurgy. Let’s examine its uses now.
What Are the Applications of Titanium?
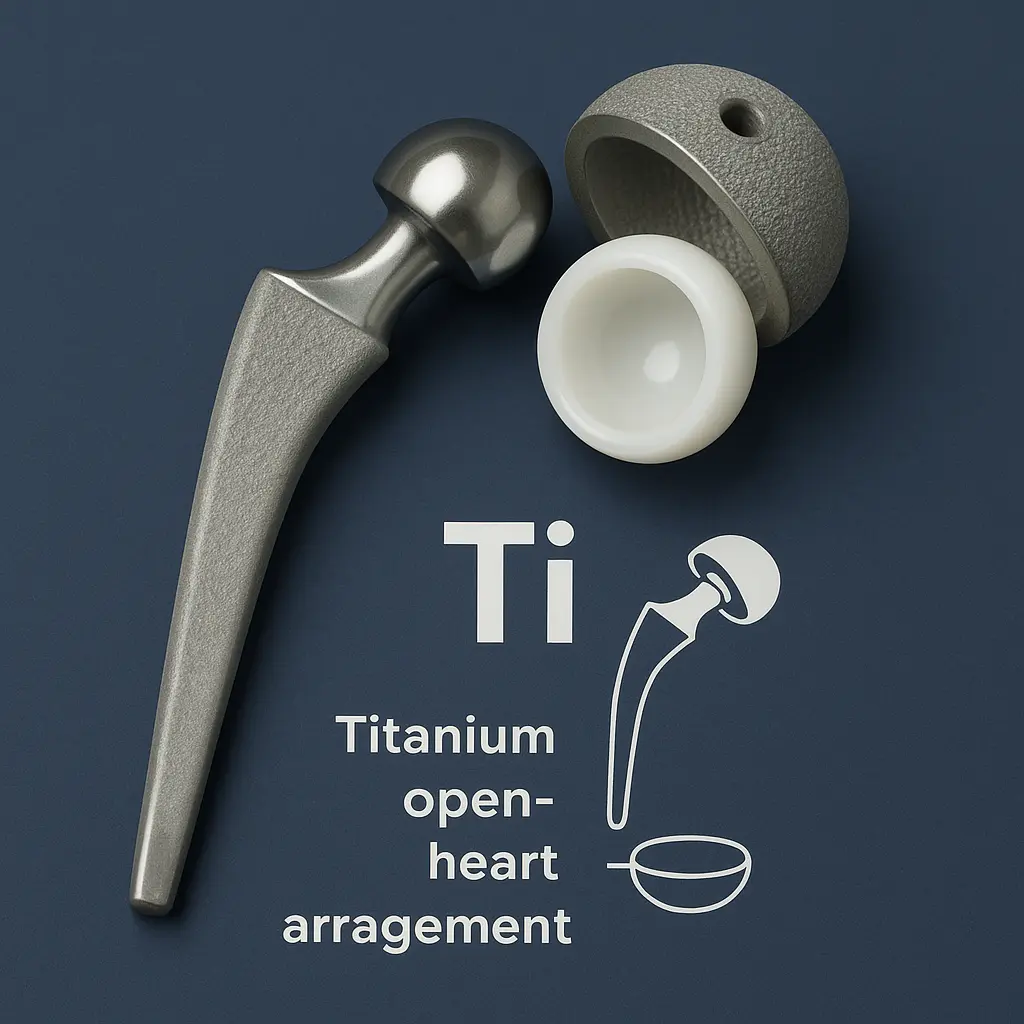
Titanium applications demonstrate its wide utility. First, it builds aircraft and spacecraft frames. These structures benefit from its strength and lightness. Titanium also enhances medical implants. Its biocompatibility suits hip replacements and screws. Additionally, it supports marine equipment. Its corrosion resistance protects underwater parts. It improves desalination plants too. It’s used in jewelry for its luster. It also crafts premium watches for durability.
Beyond industry, titanium applications include sports gear. Titanium frames make lightweight golf clubs. In electronics, it aids in high-performance batteries. It’s also used in architectural designs for durability. Titanium coatings protect surfaces from wear. Its alloys enhance bicycle frames for cyclists. However, its high cost limits mass production. For example, refining processes are energy-intensive. Research seeks cheaper methods in 2025. Thus, titanium applications are diverse yet challenging.

What Defines Titanium Chemistry?
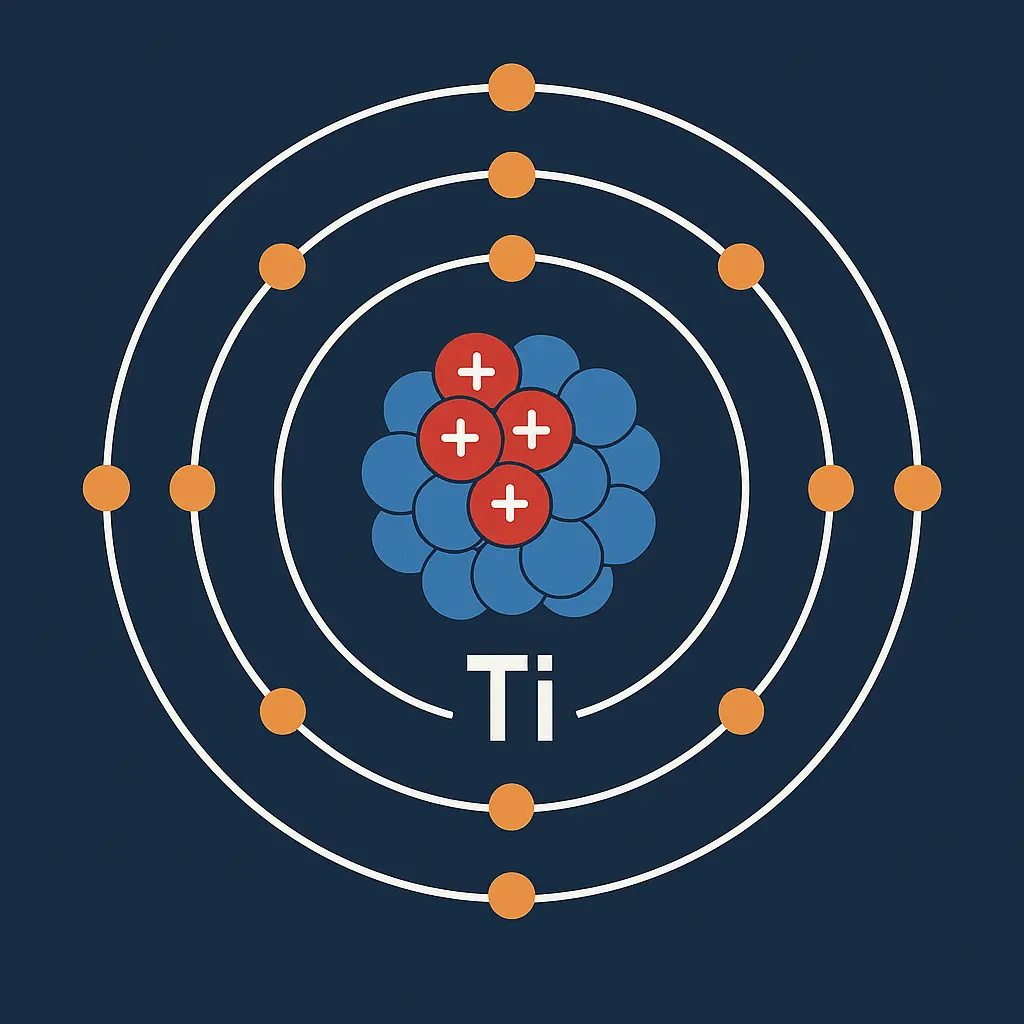
Titanium chemistry reveals intriguing behaviors. It forms a +4 oxidation state in most compounds. Titanium dioxide (TiO₂) is its stable oxide. This oxide is highly inert and white. Titanium also reacts with oxygen, forming a tough layer. Its electron configuration is [Ar] 3d² 4s². It shows moderate reactivity with air. Its compounds are often crystalline and bright. It acts as a catalyst in chemical reactions. It maintains stability at high temperatures.
Titanium chemistry shows strong oxide formation. It resists most acids, except sulfuric acid. Moreover, it forms complexes with organic molecules. These properties support pigment production. For instance, titanium dioxide colors paints. Its role in photocatalysis is growing. It also aids in water purification systems. Its use in catalytic converters is expanding. Therefore, titanium chemistry fuels advanced research.

Leave a Reply