Introduction
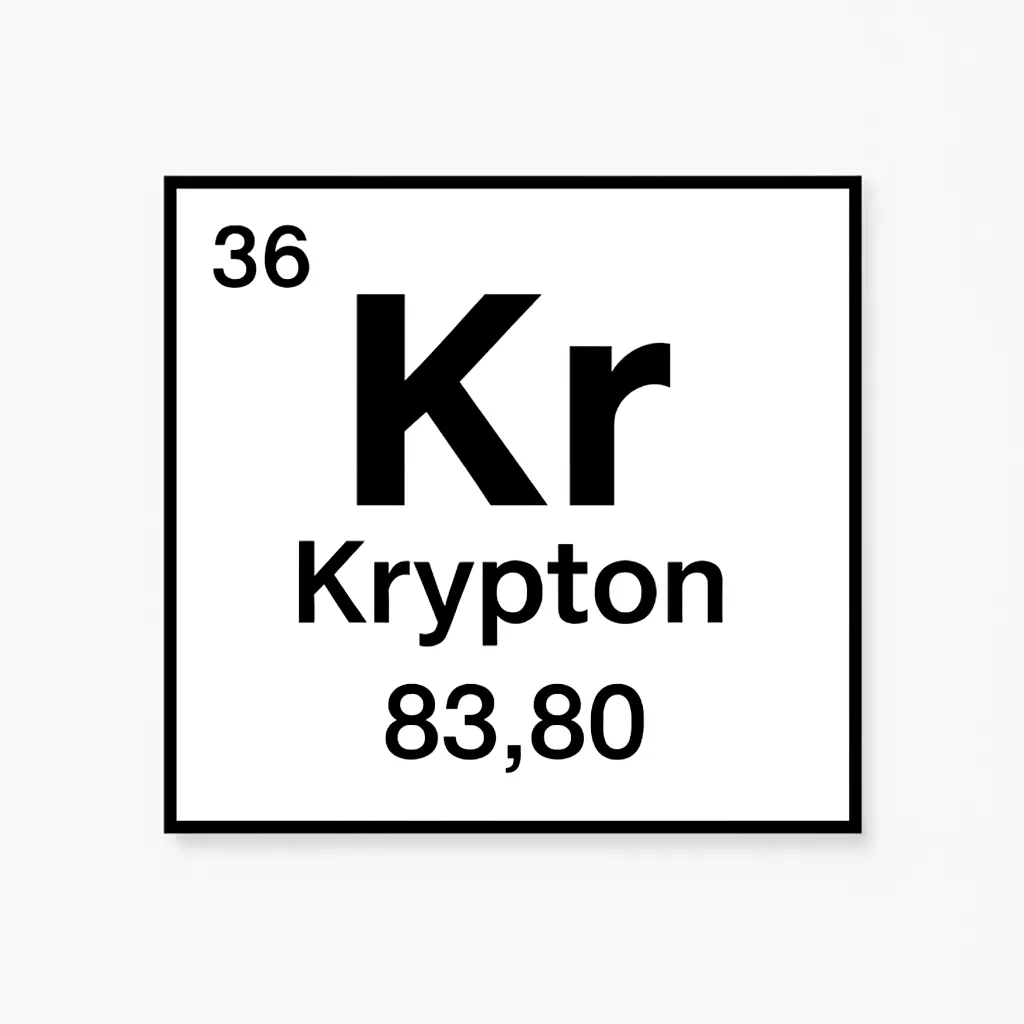
Imagine stumbling upon a gas so rare it evaded discovery for centuries. Krypton, element number 36 in the periodic table, is a colorless, odorless noble gas that quietly powers modern marvels. Unveiled in 1898, its name, from the Greek “kryptos” meaning hidden, reflects its scarce presence in Earth’s atmosphere. The Krypton element captivates with its glowing traits and surprising roles, from bright lights to space exploration. Let’s dive into its journey and why it’s a scientific gem.
Comprising just 1 part per million of our air, this elusive gas punches above its weight. Its discovery reshaped the periodic table, revealing a family of inert gases. From illuminating film studios to aiding medical scans, the Krypton element’s impact is vast despite its rarity. This article uncovers how a hidden gas became a cornerstone of chemistry and technology.
A Hidden Element Revealed
Discovered by chance in 1898, the Krypton element emerged through the work of British scientists William Ramsay and Morris Travers. While studying liquid air, they found a mysterious gas that glowed under electric current. Naming it Krypton for its elusive nature, they added a new noble gas to the periodic table. This Krypton discovery, alongside neon and xenon, was a breakthrough for chemistry. It showed how science could uncover the invisible.
Spectroscopy, a technique analyzing light patterns, helped Ramsay and Travers spot Krypton’s faint presence—only 0.0001% of Earth’s atmosphere. Their efforts earned Ramsay a Nobel Prize in 1904 for noble gas discoveries. However, Krypton’s scarcity posed challenges for early studies. The Krypton discovery proved that persistence and precision could unlock nature’s secrets. It marked a turning point in elemental science.
Traces of Krypton in the sun, detected via spectroscopy in 1868, hinted at its cosmic role before its Earth discovery. Meanwhile, the periodic table had gaps, with noble gases an unknown piece until 1898. This finding linked chemistry to the stars, expanding our view of the universe. Krypton’s story began as a cosmic clue, solved by human curiosity.
A Place in the Periodic Table
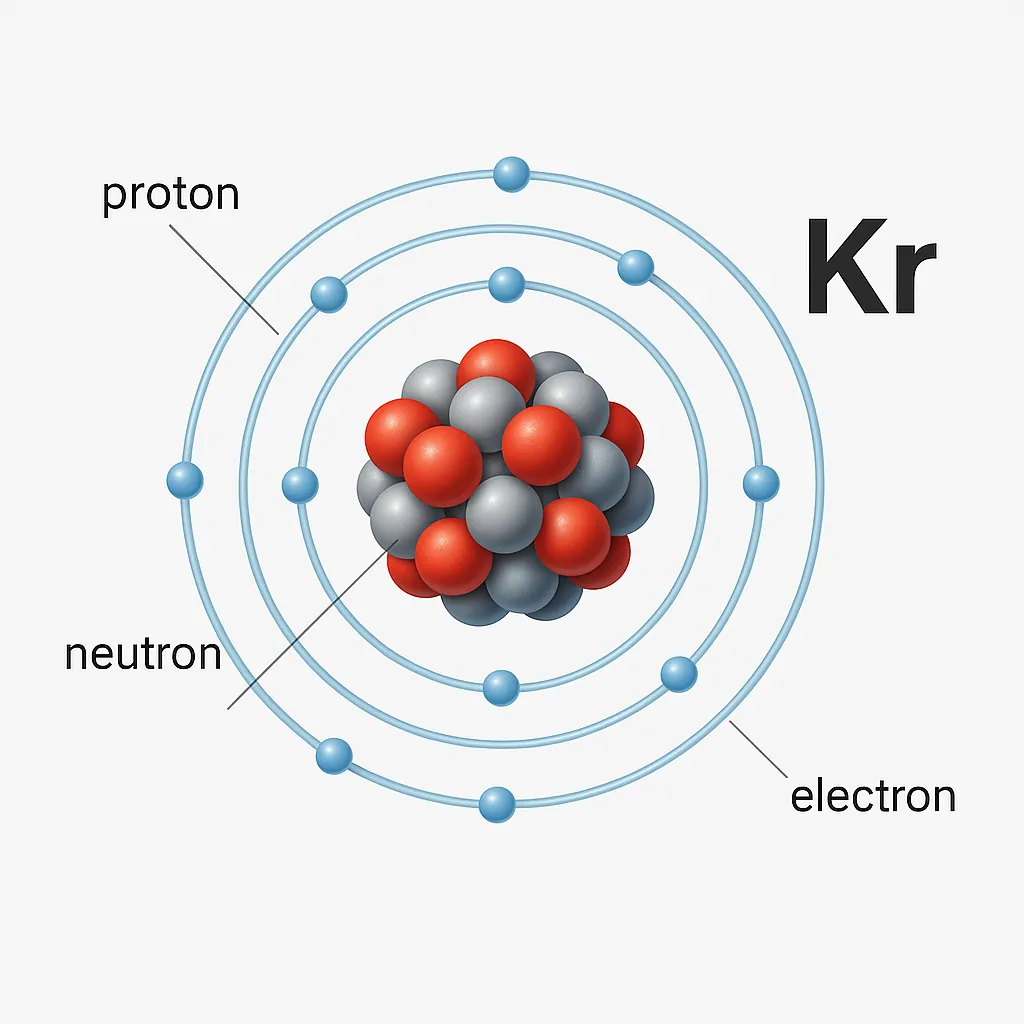
Tucked away in group 18, period 4, the Krypton element joins noble gases like helium and argon. These gases rarely react, thanks to full outer electron shells, giving them unmatched stability. This inertness defines their noble gas properties, making Krypton a chemical “loner.” The periodic table significance of Krypton lies in its role as a stable anchor for noble gas studies. It’s a vital piece of the elemental puzzle.
Boasting 36 protons and electrons, Krypton has an atomic mass of about 83.8 units. Its electron configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶) explains its resistance to bonding, though compounds like Krypton difluoride (KrF₂) form under extreme conditions. For instance, KrF₂’s discovery in 1963 showed noble gases could be reactive, defying old beliefs. This adds intrigue to Krypton’s periodic table significance, blending reliability with possibility.
Filling a gap in group 18, Krypton’s discovery completed the noble gas family, reshaping chemical theory. Denser and rarer than argon, it shares glowing traits with xenon. Its isotopes, like Krypton-86, once defined the meter, tying it to precision measurements. By connecting earthly chemistry to cosmic studies, Krypton’s place underscores its scientific weight.

Krypton’s Unique Traits
Glowing under electric charge, the Krypton element showcases noble gas properties that make it special. Invisible as a colorless, odorless gas, it emits a whitish-blue light when electrified, key to lighting applications. With a density 3.75 times heavier than air and a boiling point of -153.4°C, it stays gaseous in normal conditions. These noble gas properties suit Krypton for unique roles. It’s a subtle force with bright potential.
Resisting chemical bonds, Krypton stays stable in most environments. Yet, under high pressure or with fluorine, it forms compounds like KrF₂, proving noble gases can surprise. Additionally, isotopes like Krypton-81 help date ancient groundwater, aiding Earth science. Its presence in stars, spotted via spectroscopy, links it to stellar chemistry. The Krypton element’s blend of stability, glow, and cosmic ties fuels its fascination.
Scarcer than argon but akin to xenon in glow, Krypton’s rarity—1 part per million on Earth—contrasts with its abundance in space. For example, Krypton in meteorites helps study solar system origins. Its unique mix of earthly scarcity and cosmic presence adds mystique. These traits make Krypton a standout in both science labs and the universe.

Uses in the Modern World
Powering bright lights, Krypton applications transform our world. Krypton fills fluorescent bulbs and high-intensity lamps, producing vivid, efficient light for studios and headlights. Mixed with argon, it enhances bulb performance while keeping costs down. These Krypton applications save energy and create stunning effects, from film sets to city streets. This gas truly illuminates modern life.
In science and medicine, Krypton excels. Krypton lasers deliver precise beams for eye surgeries and research, while Krypton-83m supports lung imaging for safe diagnostics. Meanwhile, Krypton-81 dates ancient ice and water, revealing Earth’s climate past. Krypton applications in these areas drive innovation, blending technology with health and history. It’s a rare gas with remarkable reach.
Extracting Krypton, however, isn’t easy. Its scarcity requires energy-intensive air liquefaction, raising costs and environmental concerns from power use. Still, Krypton’s use in spacecraft ion thrusters, tested by NASA, aids space missions, and its presence in meteorites informs solar system studies. The Krypton element’s applications, despite extraction challenges, prove its value in advancing science and exploration.

Leave a Reply