Introduction
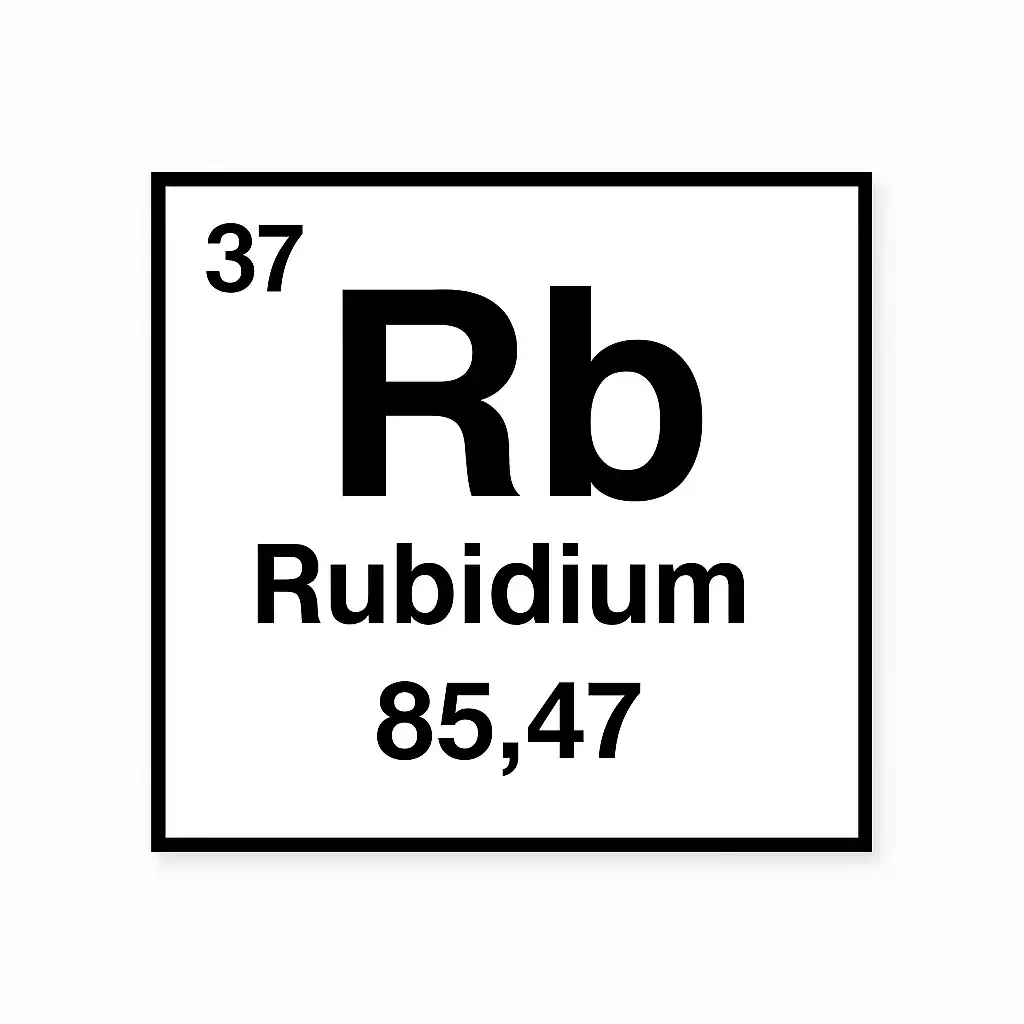
What metal can light up a lab with a fiery purple spark? Rubidium element 37, a soft, silvery alkali metal, sits quietly in the periodic table but bursts with scientific potential. Discovered in 1861, it powers atomic clocks, fuels lasers, and even colors fireworks. Found in tiny traces in Earth’s crust, rubidium’s story is one of chemistry and innovation. Let’s explore how this rare element shapes our world.
“Rubidium’s red glow opened new doors,” said chemist Gustav Kirchhoff, hinting at its discovery. Rubidium element 37 weaves a tale from 19th-century labs to modern space tech. With just 0.03% of Earth’s crust, it drives cutting-edge science and global markets. This article uncovers its history, properties, uses, and impacts, revealing why rubidium matters to teenagers and scientists alike.
Roots of Rubidium’s Discovery
Can a single flame uncover a hidden element? In 1861, Robert Bunsen and Gustav Kirchhoff found rubidium discovery history by burning lepidolite in a flame spectrometer. They spotted vivid red lines, naming it “rubidium” from the Latin “rubidus” for deep red. This marked rubidium element 37 as a breakthrough in chemistry. But pulling pure rubidium from minerals was no easy task.
Why did rubidium stay elusive? Its extreme reactivity with air and water, a key rubidium chemical properties trait, made early samples impure—Bunsen’s best was only 18% pure. By 1928, scientists like George de Hevesy used hydrolysis to isolate it, a milestone in rubidium discovery history. Miners tapped lepidolite and pollucite, but costs soared. The history of rubidium element 37 began with these challenges, setting the stage for its scientific rise.
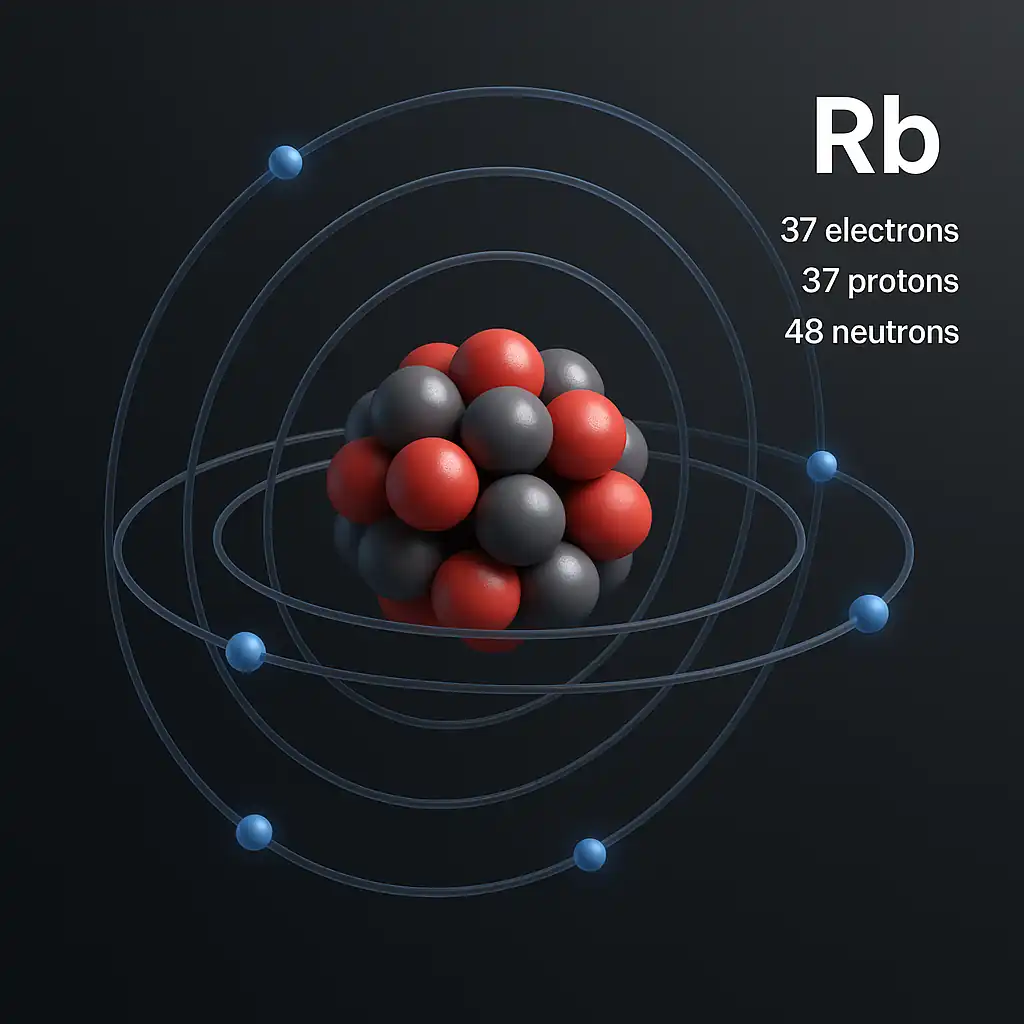
By the 1870s, labs across Europe studied rubidium’s isotopes: 72% stable Rb-85 and 28% radioactive Rb-87, with a 48.8-billion-year half-life. This slight radioactivity, enough to fog film in 110 days, fascinated chemists. These early steps shaped how we understand rubidium chemical properties, from its soft texture to its explosive nature.
Spread of Rubidium’s Applications
Step into a 1900s lab buzzing with experiments. Rubidium chemical properties, like a low 39.3°C melting point and water reactivity, made it a research gem. Unlike sodium, rubidium element 37 sinks in water due to its 1.53 g/cm³ density. Early uses included vacuum tubes, where it trapped oxygen, and glassmaking, where it boosted durability, as seen in lab equipment.
Could rubidium light up technology? By the 1960s, rubidium uses in technology grew, with rubidium chloride aiding DNA studies in biology labs. Its rarity made it a biomarker, while rubidium nitrate sparked purple fireworks, tying the cultural impact of rubidium to festivals in Japan and the U.S. Yet, rubidium production challenges, like scarce minerals and $1200/kg costs, kept it from mass markets.
In the 1980s, rubidium found a star role in atomic clocks. Rb-87’s precise electron jumps powered GPS satellites, syncing time to a second per million years. But limited mine outputs, mostly from Canada and Namibia, posed rubidium production challenges. Rubidium element 37 became a niche hero, driving science despite its high price and tricky handling.

Scientific Innovations with Rubidium
What if a metal could measure time with cosmic precision? Rubidium uses in technology peaked with atomic clocks, where Rb-87’s vibrations ensure GPS accuracy to within a second over a million years. Since the 1990s, rubidium element 37 has synced internet networks and satellites. However, cesium clocks outshine it in precision, a hurdle for rubidium in top-tier tech.
In 1995, rubidium rewrote physics. Using its atoms, scientists created a Bose-Einstein condensate, chilling rubidium to near-absolute zero where atoms barely move. This Nobel Prize-winning feat, tied to rubidium chemical properties, fueled quantum computing research. Labs in Germany and the U.S. now use rubidium in laser cooling, slowing atoms to study their quantum dance.
Today, rubidium drives ion engines for spacecraft, though cesium is cheaper. Rubidium iodide powers thin-film batteries, a $500 million market. Yet, rubidium production challenges, like air-igniting risks requiring argon storage, limit growth. Rubidium element 37 pushes science forward, balancing bold innovations with practical constraints.

Scientific and Cultural Impact
“Rubidium keeps our satellites on track,” notes a NASA scientist, highlighting its role in atomic clocks. Rubidium element 37 shapes science, from GPS guiding global travel to Rb-87 dating rocks 4.5 billion years old in geology. Its precision aids physics, with Bose-Einstein condensates unlocking quantum secrets. The cultural impact of rubidium lies in its quiet presence in labs worldwide.
Rubidium also colors human moments. Its purple flame in fireworks dazzles at events like Canada’s Victoria Day or China’s Lunar New Year, boosting the cultural impact of rubidium. In medicine, rubidium-82 maps heart conditions, detecting blockages with 90% accuracy. But its radioactivity demands strict safety, a challenge for hospitals and researchers.
The race for rubidium, mined from Canada’s pollucite or recycled from Australia’s lithium waste, faces rubidium production challenges like environmental runoff and $1200/kg costs. Programs like the USGS promote safer mining, cutting waste by 20%. Showcased at events like London’s Science Museum exhibits, rubidium element 37 blends innovation with cultural sparks.

Leave a Reply