Introduction
What is carbon, and why is it so critical? This element, with atomic number 6, is a non-metal in the periodic table. It forms countless compounds, serving as the foundation of life. It exists in DNA, proteins, and carbohydrates, vital for all organisms. The element also fuels industries like energy and nanotechnology. It makes up 0.2% of the Earth’s crust and appears as carbon dioxide in the air. This article covers the element’s properties, uses, natural cycles, and sustainability challenges. It highlights the element’s profound role in our world.
Chemical and Physical Properties
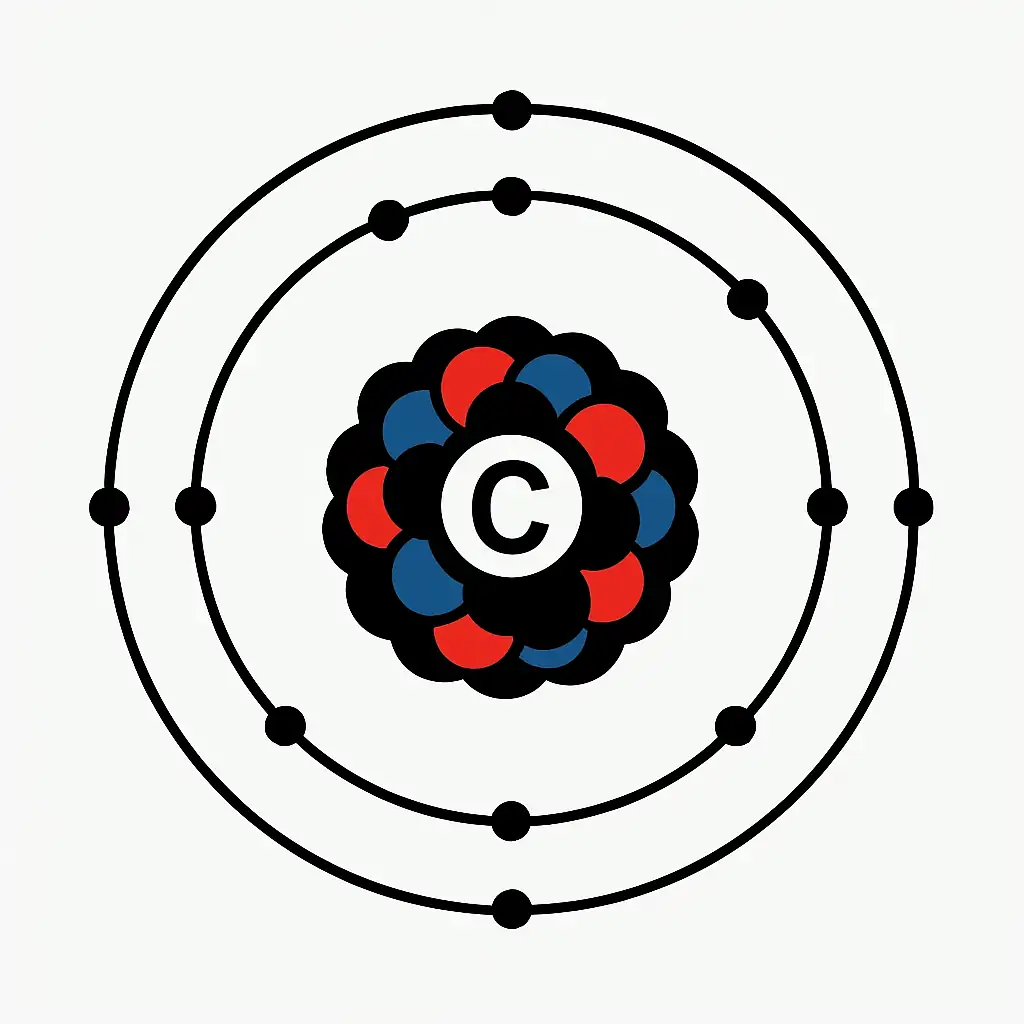
What is carbon in its chemical structure? This element has multiple allotropes with distinct traits. What is carbon physically? Diamond is the hardest natural substance, conducting heat five times better than copper. Graphite, arranged in layers, is soft and conductive, ideal for batteries. Amorphous forms, like coal, are used as fuel. The element’s atomic mass is 12.01 u. Its electron configuration (1s² 2s² 2p²) supports four covalent bonds. This forms chains, rings, and complex molecules. Diamond melts above 3,550°C. The material resists corrosion. It bonds with hydrogen and oxygen, creating compounds like methane and plastics. Its versatility drives natural and synthetic systems.
Key Applications
What is carbon in modern industry? This element enables diverse applications. What is carbon in energy systems? Hydrocarbons like oil and gas supply 80% of global energy, though renewables are growing. What is carbon in manufacturing? Steel, alloyed with iron, is essential for construction. Graphite electrodes produce 30% of global steel in arc furnaces. Lightweight fibers strengthen aerospace parts, vehicles, and wind turbines. Graphene, a single graphite layer, provides high conductivity for transistors and screens. Nanotubes, 100 times stronger than steel, are tested for drug delivery and cables. Activated forms purify water, removing 99% of impurities. Diamonds cut industrial tools. Urea fertilizer boosts food production. These applications show the element’s transformative power.

Carbon Cycle in Nature
What is carbon in Earth’s balance? The cycle moves this element through air, land, oceans, and life. What is carbon in plant processes? Plants absorb 120 gigatons of carbon dioxide yearly via photosynthesis, producing glucose and oxygen. Animals consume plants, releasing the element through respiration. Decomposition returns it to soil, forming fossil fuels over millions of years. Oceans absorb 25% of atmospheric carbon dioxide, creating carbonates. Volcanoes and weathering release stored amounts, balancing the cycle. Human activities add 9 gigatons yearly, disrupting this balance. Deforestation reduces sinks, raising atmospheric levels. This drives 70% of global warming. Restoring forests and oceans can stabilize the cycle.

Challenges and Sustainability
What is carbon in environmental concerns? Emissions from fossil fuels cause climate change. Global temperatures have risen 1.1°C since pre-industrial times. What is carbon in industrial impacts? Burning hydrocarbons releases 36 gigatons of carbon dioxide yearly. Cement production contributes 8% of emissions. Methane from livestock adds to the issue. Renewables provided 29% of electricity in 2024, but scaling is slow. Capture technologies trap 90% of plant emissions, costing $50–100 per ton. Deforestation has cut 15% of forests since 2000, reducing sinks. Only 9% of plastic is recycled globally. Bio-based materials and carbon-negative concrete are emerging. Scaling these requires funding. Sustainable practices are crucial for managing this element.

Leave a Reply