Introduction
Oxygen, the eighth element in the periodic table, is a colorless, odorless gas we all depend on. It makes up 21% of the air we breathe, supporting life and fueling fires. Understanding how many electrons does oxygen have reveals why it’s so versatile in nature and industry. Discovered in 1774 by Joseph Priestley, oxygen is vital for everything from human breathing to rocket launches. For example, it combines with hydrogen to form water, the basis of life. This article explores oxygen’s properties, its role in the environment, its many uses, and how it’s produced, showing why it’s essential to our world.

Chemical and Physical Properties
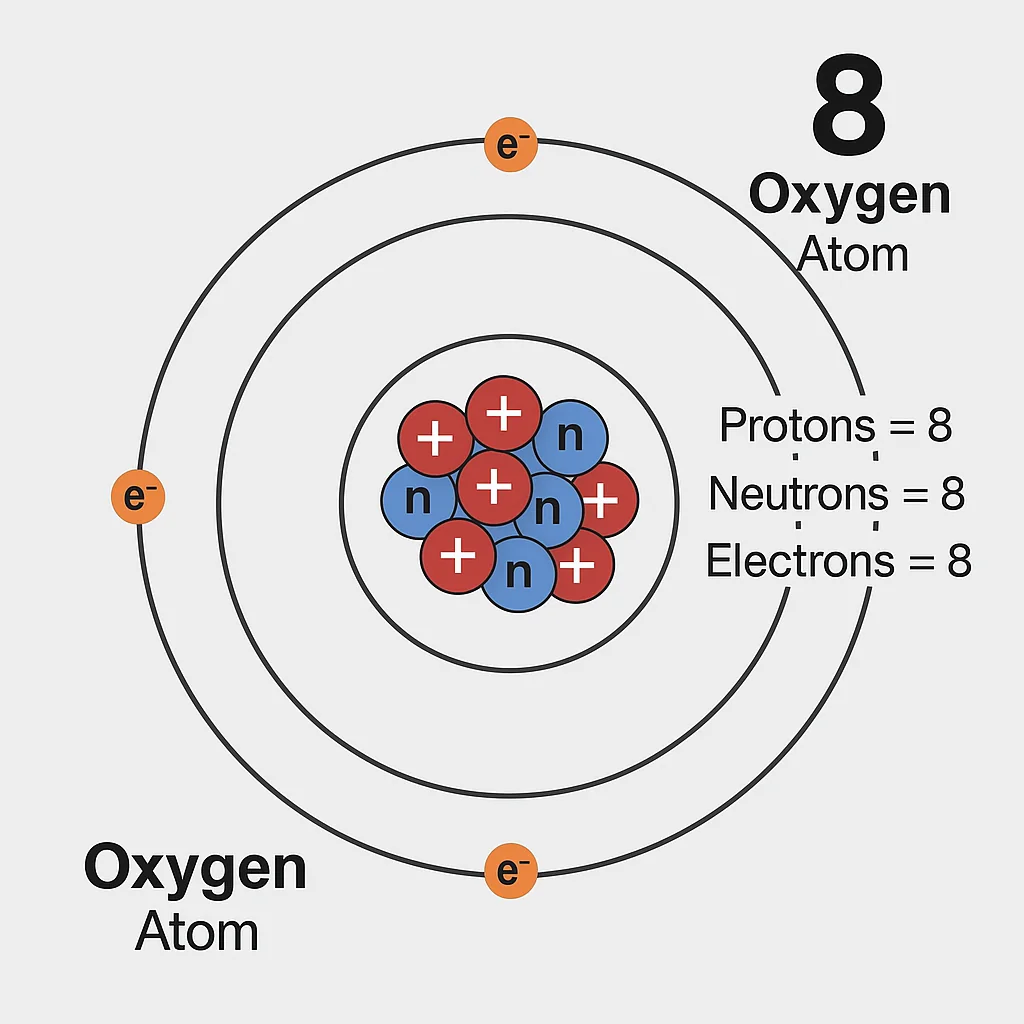
Oxygen exists as a diatomic gas, O₂, with a strong double bond. Its atomic mass is 15.999 u, and it possesses eight electrons, as answered by the question, how many electrons does oxygen have. These electrons, arranged as 1s² 2s² 2p⁴, include six in the outer shell, driving oxygen’s ability to bond with other elements. How many electrons does oxygen have in its reactive form? With eight electrons, it seeks two more to become stable, making it highly reactive. At room temperature, oxygen is a gas, turning into a pale blue liquid at -218.8°C. Its boiling point is -183°C, and its density, 1.429 g/L, is slightly heavier than air. For instance, liquid oxygen powers space missions. This reactivity requires careful handling but enables countless applications.

Role in Nature
Oxygen is a cornerstone of Earth’s ecosystems. It forms 46% of the planet’s crust, mostly in compounds like silica. In the air, it enables animals and humans to breathe. Plants release oxygen through photosynthesis, producing 120 billion tons yearly. How many electrons does oxygen have in water molecules? Its eight electrons create strong bonds in H₂O, shaping oceans and weather. Oxygen also forms the ozone layer, blocking 99% of harmful UV rays. However, too much oxygen can harm cells, causing issues for divers at high pressures. For example, divers adjust oxygen levels to stay safe. The oxygen cycle keeps atmospheric levels steady, supporting life and ecological balance. This cycle ensures nature thrives.

Applications
Oxygen’s versatility drives numerous applications. In medicine, it is used for oxygen therapy, aiding patients with respiratory issues, with 1 million treatments annually in the US. In industry, oxygen enhances combustion in steel production, cutting energy costs by 20%. Liquid oxygen powers rocket engines, used in 90% of space launches. How many electrons does oxygen have in these processes? Its eight electrons ensure reactivity for fuel combustion. In environmental systems, oxygen treats wastewater, breaking down 95% of organic pollutants. Oxygen is also used in welding, chemical synthesis, and food preservation. For instance, it extends shelf life in packaged foods. Emerging uses include oxygen-based fuel cells for clean energy, reducing emissions by 30%. These applications highlight oxygen’s broad utility.
Production and Extraction
Most oxygen comes from fractional distillation of liquid air. Air is cooled to -200°C, liquefied, and separated to isolate oxygen with 99.5% purity. This process supplies 200 billion cubic meters yearly. It’s energy-intensive, using 0.5–1 kWh per cubic meter. How many electrons does oxygen have in its pure form? Its eight electrons remain consistent, ensuring its chemical properties. Smaller-scale methods, like pressure swing adsorption, provide medical oxygen, meeting 10% of demand. Electrolysis of water produces oxygen too, used in 5% of cases. Major producers include the US, China, and Germany. Liquid oxygen is stored in insulated tanks to avoid evaporation. For example, hospitals rely on steady supplies. New membrane technologies aim to lower costs, making oxygen more accessible.

Leave a Reply