Introduction
Chlorine element uses are widespread in industry and daily life. As element 17, chlorine is a powerful halogen. It disinfects water, produces plastics, and supports chemical reactions. Additionally, its unique properties make it essential. As of May 2025, chlorine’s importance continues to grow. This article explores chlorine element uses in depth. It covers its place in the periodic table, properties, applications, and safety. Thus, you’ll see why chlorine is vital.
Chlorine was identified by Carl Wilhelm Scheele in 1774. Sir Humphry Davy confirmed it as an element in 1810. For clarity, 1 ppm equals 0.0001%. Also, chlorine’s atomic weight is 35.45. It’s found in nature as sodium chloride in seawater. Industries rely on it for sanitation and manufacturing. This guide highlights chlorine’s impact on modern life. It’s a lifesaver but also a potential hazard. How does chlorine affect your routine? Let’s dive in.

Chlorine in Periodic Table: A Halogen’s Role
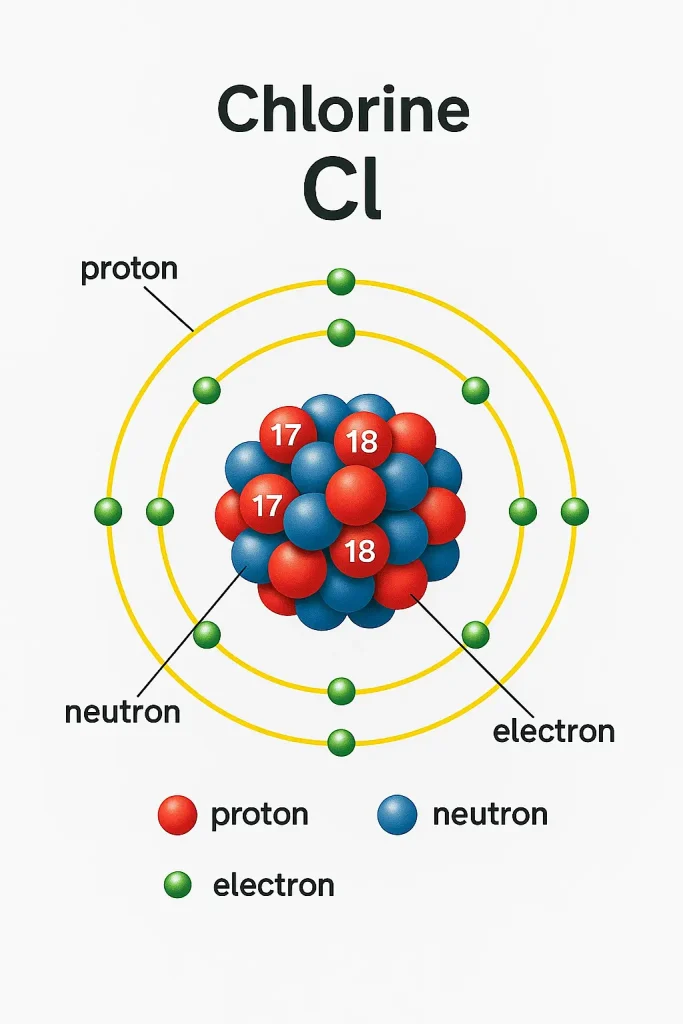
Chlorine in periodic table sits in Group 17, Period 3. It’s the second-lightest halogen, between fluorine and bromine. Thus, it shares traits with other halogens like iodine. Its atomic number is 17, meaning 17 protons and electrons. Additionally, its electron configuration is [Ne]3s²3p⁵. This setup makes it highly reactive. Moreover, chlorine in periodic table is a nonmetal. It rarely exists alone due to its reactivity.
Chlorine is often found as sodium chloride, or table salt. Seawater contains about 1.9% chlorine as chloride ions. Also, minerals like halite are rich sources. As a result, chlorine in periodic table helps understand halogens. It has a high electron affinity, second to fluorine. This makes it a strong oxidizing agent. Furthermore, its electronegativity is 3.16 on the Pauling scale. Consequently, chlorine’s position shapes its chemical behavior. It’s key in both nature and science.
Chlorine Chemical Properties: Reactivity and Behavior
Chlorine chemical properties define its versatility. It’s a yellow-green gas with a pungent smell. You can detect it at 3.5 ppm by odor. Its melting point is -101.5°C, and it boils at -34.04°C. Also, chlorine has a density of 3.2 g/L, heavier than air. This causes it to sink if released. Moreover, chlorine chemical properties include high reactivity. It combines with most elements except noble gases.
Chlorine has seven valence electrons, needing one for stability. It often forms a -1 oxidation state in compounds. However, it can show +1, +5, or +7 states. For example, in hypochlorous acid, it’s +1. Additionally, chlorine reacts with water to form hydrochloric acid. This reaction is key for disinfection. As a result, chlorine chemical properties make it a strong oxidizer. It’s widely used in chemical processes. Understanding these traits is key to safe use.

Chlorine Element Uses in Industry and Daily Life
Chlorine element uses are critical in various sectors. It’s widely known for disinfecting drinking water and pools. Thus, it kills bacteria, making water safe to drink. In the US, chlorine has been used for this since 1908. Additionally, it’s used in wastewater treatment. About 85% of pharmaceuticals rely on chlorine during production. Moreover, chlorine element uses include making polyvinyl chloride (PVC). PVC is used in pipes, window frames, and medical tubing.
Chlorine is vital in the paper and textile industries. It bleaches pulp for white paper products. Also, it’s used in producing dyes, solvents, and insecticides. As a result, chlorine element uses support many consumer goods. It’s involved in making antiseptics, paints, and plastics. Furthermore, chloride ions from salt are dietary essentials. Humans need about 3 grams of chloride daily. In agriculture, chlorine disinfects soil and protects crops. Consequently, chlorine element uses impact health and industry. Its versatility makes it indispensable in modern life.

Chlorine Safety Concerns: Handling and Risks
Chlorine safety concerns arise due to its toxicity. It’s a respiratory irritant, even at low levels. Thus, 1,000 ppm can be fatal within minutes. Chlorine gas was used as a chemical weapon in World War I. Additionally, liquid chlorine burns the skin on contact. Its high density causes it to sink, posing risks in enclosed spaces. Moreover, chlorine safety concerns include accidental leaks. Industrial accidents can release large amounts into the air.
Proper handling reduces risks significantly. Chlorine should be stored in well-ventilated areas. Workers need protective gear like masks and gloves. Also, the EPA regulates its use to protect public health. Furthermore, chlorine reacts with organic matter to form harmful byproducts. For example, trihalomethanes in water may pose health risks. In pools, over-chlorination irritates eyes and skin. Additionally, chlorine’s reaction with CFCs in the stratosphere depletes ozone. As a result, chlorine safety concerns require strict guidelines. Monitoring exposure levels is crucial.

Leave a Reply