Introduction
Ever heard of potassium element uses? It’s a super cool topic! Potassium, element 19, shines as a reactive metal. This element powers your body, boosts crops, and aids industries. As of May 2025, it remains a key player in science, health, and farming. Therefore, this article dives deep into potassium element uses. We’ll explore its spot in the periodic table, its properties, how it was discovered, and its applications. Ready to learn something new? Let’s jump in!
Potassium plays a big role in nature. For example, it’s found in your food, soil, and cells! About 2.6% of Earth’s crust holds potassium. So, how does it shape our lives? Stick around, and I’ll reveal why this metal rocks.
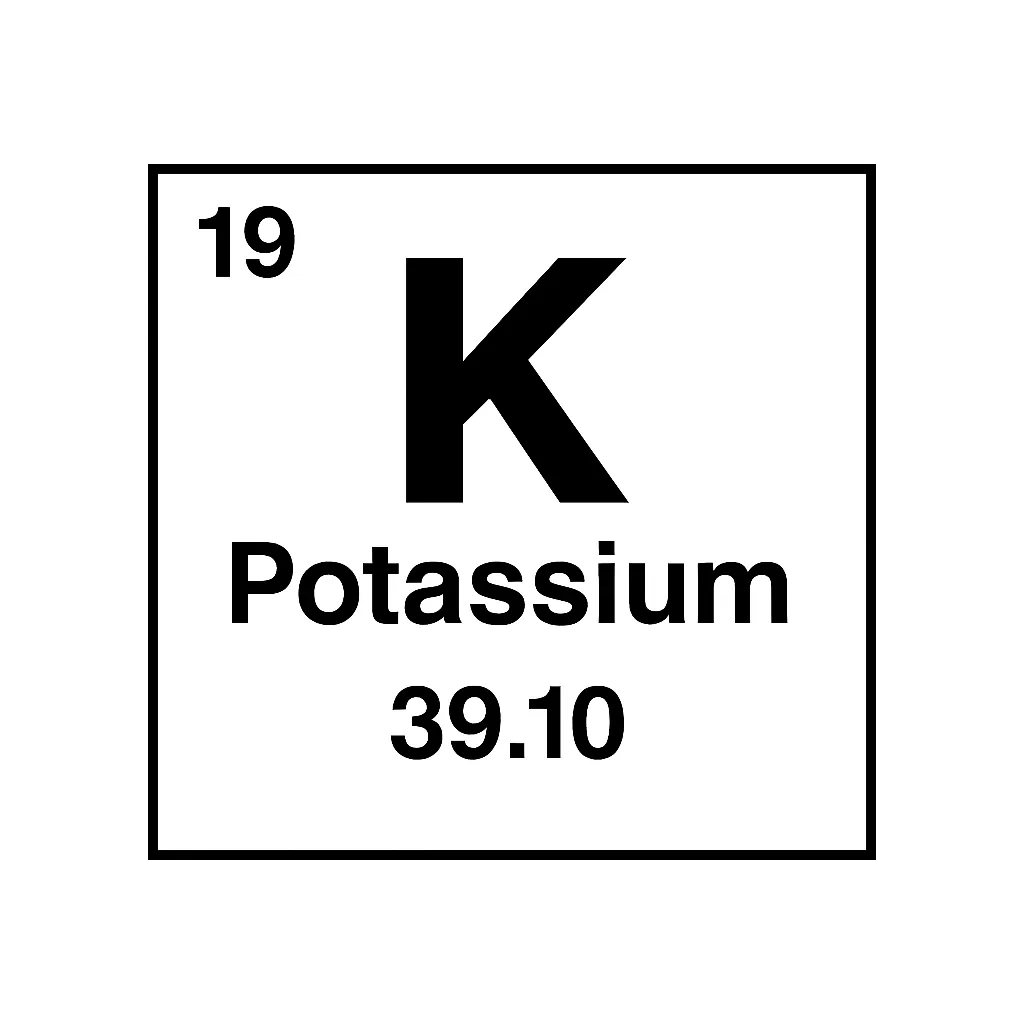
Where Does Potassium Fit in the Periodic Table?
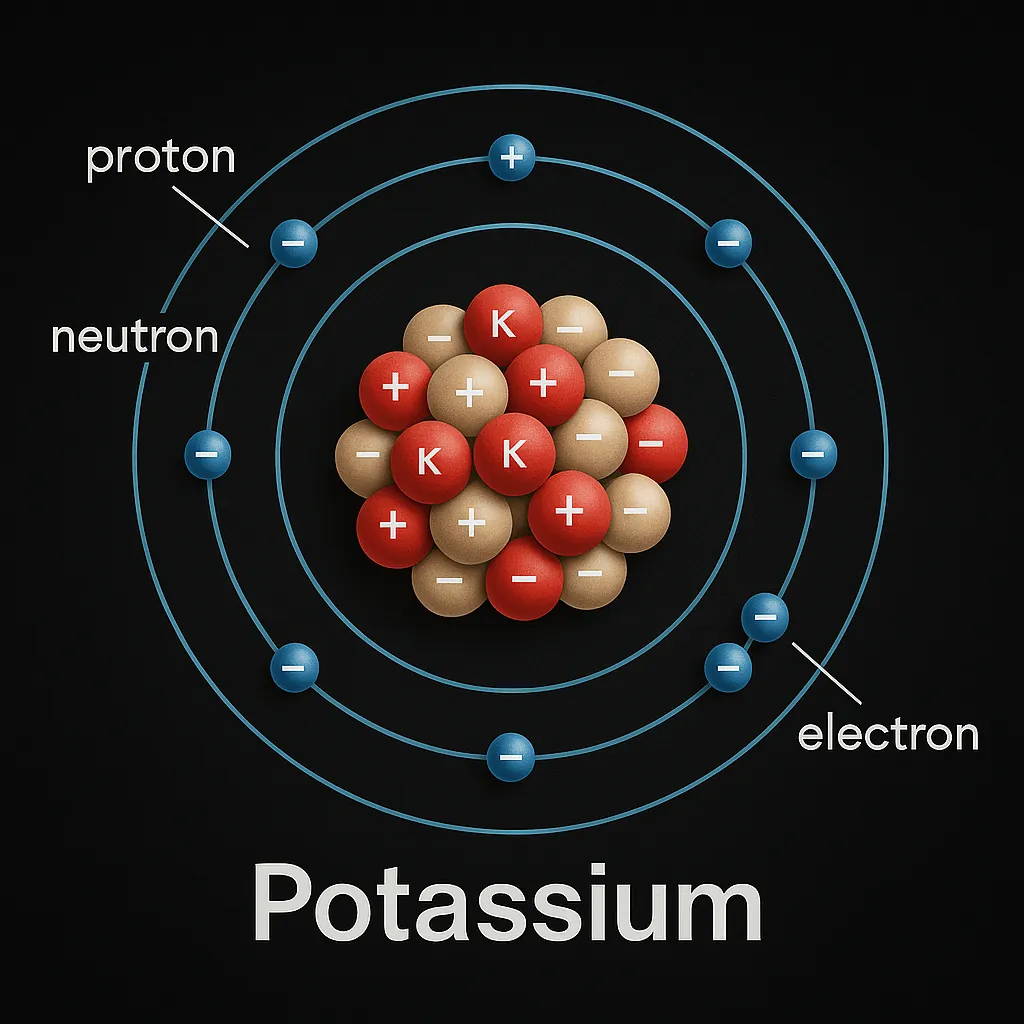
Potassium in periodic table sits in Group 1, alongside alkali metals like lithium and sodium. That means it has 19 protons and 19 electrons. Its electron setup, [Ar]4s¹, makes it super reactive. It’s always eager to lose that one outer electron and bond. Besides, it lands in Period 4, just below sodium, sharing traits with extra punch.
It’s not the most common element, yet it’s still present—about 2.6% of Earth’s crust by weight. Potassium in periodic table shows up mostly as potassium-39, which stays stable. Additionally, potassium-40, a rare radioactive isotope, decays into argon over millions of years. That process helps date old rocks! Thus, its reactivity drives its usefulness. Let’s explore its properties next to see why it stands out.
What Are Potassium’s Properties Like?
Potassium properties are honestly pretty wild. This soft, silvery metal cuts easily with a butter knife! Reacting crazily with water, it releases hydrogen gas and heat. In fact, a small explosion with sparks can follow this reaction. Stored under oil or kerosene, safety is ensured. Meanwhile, conducting electricity when molten, it proves handy in some applications. Oxidizing quickly in air, an oxide layer forms on its surface. Hence, keeping it in oil or kerosene prevents mishaps. Let’s dig into how we discovered this fiery metal next!

How Was Potassium Discovered?
Potassium discovery history dates back to 1807. A British chemist, Humphry Davy, was the genius behind it. He used electrolysis on potash—potassium carbonate—to split it. Passing an electric current through it, he isolated potassium metal and oxygen gas. That marked a huge moment, being the first time anyone saw pure potassium!
Before Davy, people knew potash from wood ashes, using it for soap and glass. Yet, no one realized it hid a new element. So, potassium discovery history proved it’s an alkali metal, just like sodium. Davy’s work opened doors for finding other metals this way. Then, he even discovered sodium right after! Consequently, his experiments earned him a huge name in science. How awesome is that? Now, let’s see what potassium does for us today.

What Are Potassium Element Uses and Safety Tips?
Applications of potassium element are all around us, and they’re super important. First, it shines in fertilizers—about 90% of mined potassium goes there. Often in potassium chloride or sulfate form, it helps plants grow strong roots and resist drought. Farmers rely on it for crops like bananas, potatoes, and oranges. Besides, your body benefits greatly too. Potassium ions keep nerves firing, muscles contracting, and your heart beating steady. You get it from foods like bananas, avocados, and spinach—adults need about 3,400 mg daily!
Industry taps this metal for making glass, soaps, and detergents. Meanwhile, potassium hydroxide steps up for liquid soaps and some batteries. Potassium-ion batteries are gaining traction in 2025 as a cheaper alternative to lithium-ion ones. Researchers see big potential in energy storage with these. Also, doctors use potassium chloride in IV drips to balance electrolytes in patients. However, safety’s key—reacting violently with water, it sparks and burns if wet! It catches fire in air, so store it in oil or kerosene. Skin contact causes burns due to moisture reactions, so always handle with gloves. Use a controlled setting to stay safe. Therefore, potassium element uses are amazing, but careful handling prevents accidents.

Leave a Reply