Introduction
Aluminum, a shiny silver metal, is one of the most versatile elements on Earth. It’s the third most abundant element in Earth’s crust, making up about 8% of its weight. With the chemical symbol Al and atomic number 13, aluminum has transformed industries worldwide. This lightweight metal was once more valuable than gold due to its rarity. Today, its applications are endless and touch nearly every part of our lives.
From soda cans to airplane bodies, aluminum’s uses are all around us. It’s even in the foil you use to wrap leftovers! As of May 2025, the demand for aluminum keeps growing due to its unique properties. This article dives into aluminum evolution, focusing on the applications of aluminum. We’ll explore how it formed, its properties, and its role in our world today.

The Discovery of Aluminum
Aluminum evolution began long before humans could use it widely. In 1825, Danish scientist Hans Christian Ørsted first isolated aluminum from a compound called alum, which was used in ancient times for dyeing fabrics. At first, extracting aluminum was incredibly difficult because it bonds tightly with oxygen in nature. As a result, it was rare and very expensive—more costly than silver or gold.
For example, in the 1850s, Napoleon III of France used aluminum for fancy dinnerware to impress his guests, showing off its value. However, everything changed in 1886 when two young scientists—Charles Martin Hall in the U.S. and Paul Héroult in France—developed a new method called electrolysis. This process used electricity to separate aluminum from its ore, bauxite, making it much cheaper. Consequently, the applications of aluminum became accessible to industries, sparking a revolution in manufacturing and construction that continues to this day.
Aluminum’s Journey to Earth
Aluminum didn’t originate on our planet—it came from the stars. Billions of years ago, aluminum formed in massive stellar explosions called supernovae, where intense heat and pressure fused lighter elements into heavier ones. These explosions scattered aluminum atoms across space, spreading them into cosmic clouds.
Then, about 4.6 billion years ago, our solar system formed from one such cloud of gas and dust. Aluminum settled into Earth’s crust as the planet took shape during its molten early years. On Earth, aluminum is never found in pure form because of its reactivity. Instead, it’s locked in minerals like bauxite, which is the main source of aluminum today.
Over millions of years, geological processes like weathering and erosion concentrated bauxite deposits in tropical regions, such as Australia, Guinea, and Brazil. Australia alone produces about 30% of the world’s bauxite, according to 2023 data. Consequently, the applications of aluminum depend on these natural deposits, which are mined to meet global demand.

Properties of Aluminum
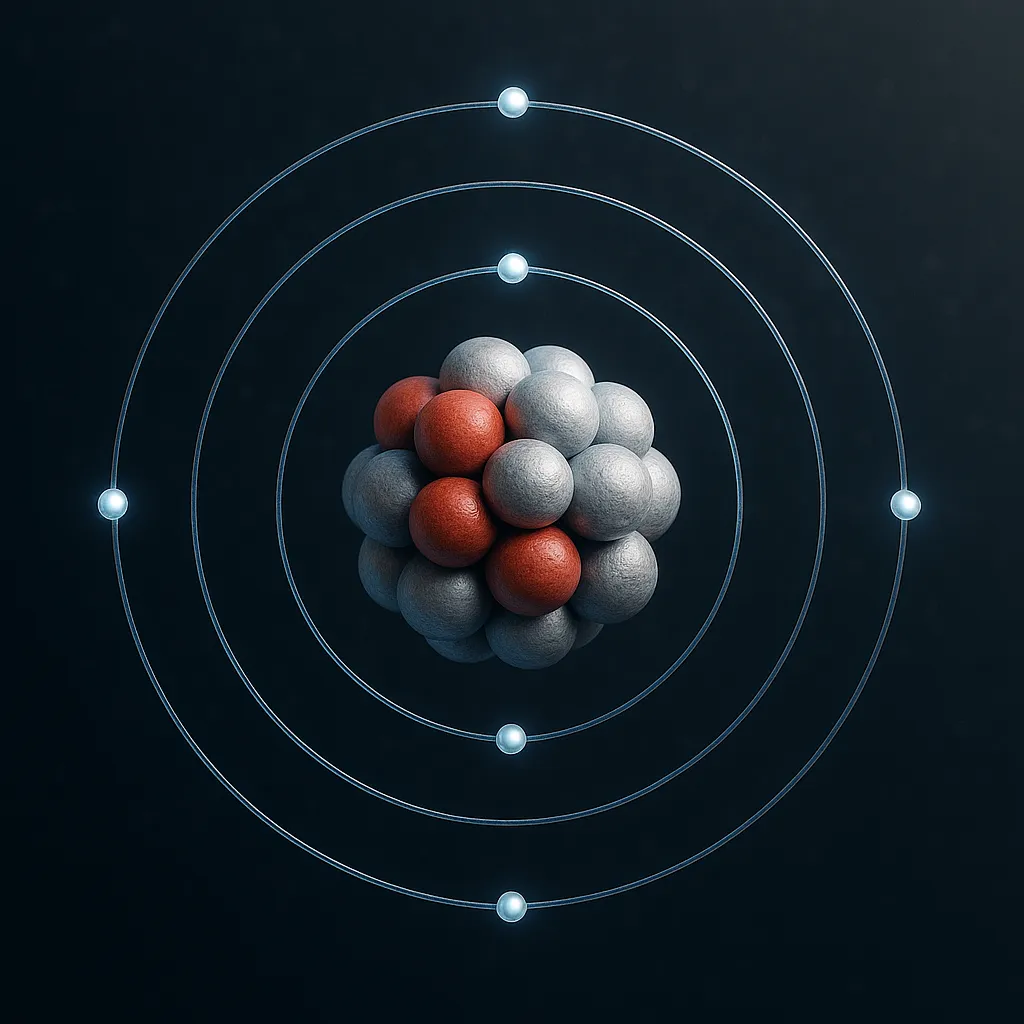
The applications of aluminum are closely tied to its remarkable properties. Aluminum has an atomic mass of 26.98 and belongs to group 13 in the periodic table, also known as the boron group. At room temperature, it’s a solid with a bright, silvery appearance that reflects light beautifully. One of its standout traits is its light weight—it’s about one-third the weight of steel, making it ideal for reducing weight in products.
Meanwhile, the properties of aluminum include excellent resistance to corrosion. A thin oxide layer forms naturally on its surface, protecting it from rust even in harsh environments like salty ocean air. Aluminum is also a fantastic conductor of heat and electricity, which is why it’s used in power lines to transmit electricity over long distances.
For instance, many electrical grids rely on aluminum cables instead of copper because it’s cheaper and lighter. In addition, aluminum is flexible, non-magnetic, and easy to shape, which makes it perfect for creating thin foils or intricate car parts. These properties of aluminum make it a go-to material for industries ranging from aerospace to packaging.

Aluminum in Our World Today
The applications of aluminum are truly everywhere, shaping the modern world in countless ways. In 2023, Boeing used aluminum to build the body of its 737 aircraft, a popular commercial plane. Its light weight helps planes save fuel while maintaining the strength needed to carry passengers safely across the globe. Similarly, aluminum is the star of the beverage industry. Billions of soda cans are made each year, and most are recycled due to aluminum’s recyclability.
This ties into the importance of recycling aluminum, which uses 95% less energy than producing new aluminum from bauxite. For example, recycling one aluminum can saves enough energy to power a TV for three hours! This process also helps reduce waste, making aluminum and the environment closely connected. Meanwhile, aluminum in vehicle manufacturing is on the rise.
The Ford F-150 truck, one of America’s best-selling vehicles, uses an aluminum body to be lighter and more fuel-efficient, cutting down on emissions. Electric cars are taking this further—companies like Tesla are using more aluminum to extend battery range by reducing vehicle weight. In construction, aluminum is used for window frames, roofing, and building panels because it doesn’t rust, even after years of exposure to rain.
At home, aluminum foil keeps food fresh, and aluminum cookware conducts heat evenly for better cooking. However, mining aluminum has challenges. Bauxite mining can harm ecosystems, and the refining process requires a lot of energy. On the other hand, advancements in sustainable mining and a focus on recycling aluminum are helping reduce its environmental impact. These applications of aluminum show how versatile and essential this metal has become in our daily lives.

Leave a Reply