Introduction
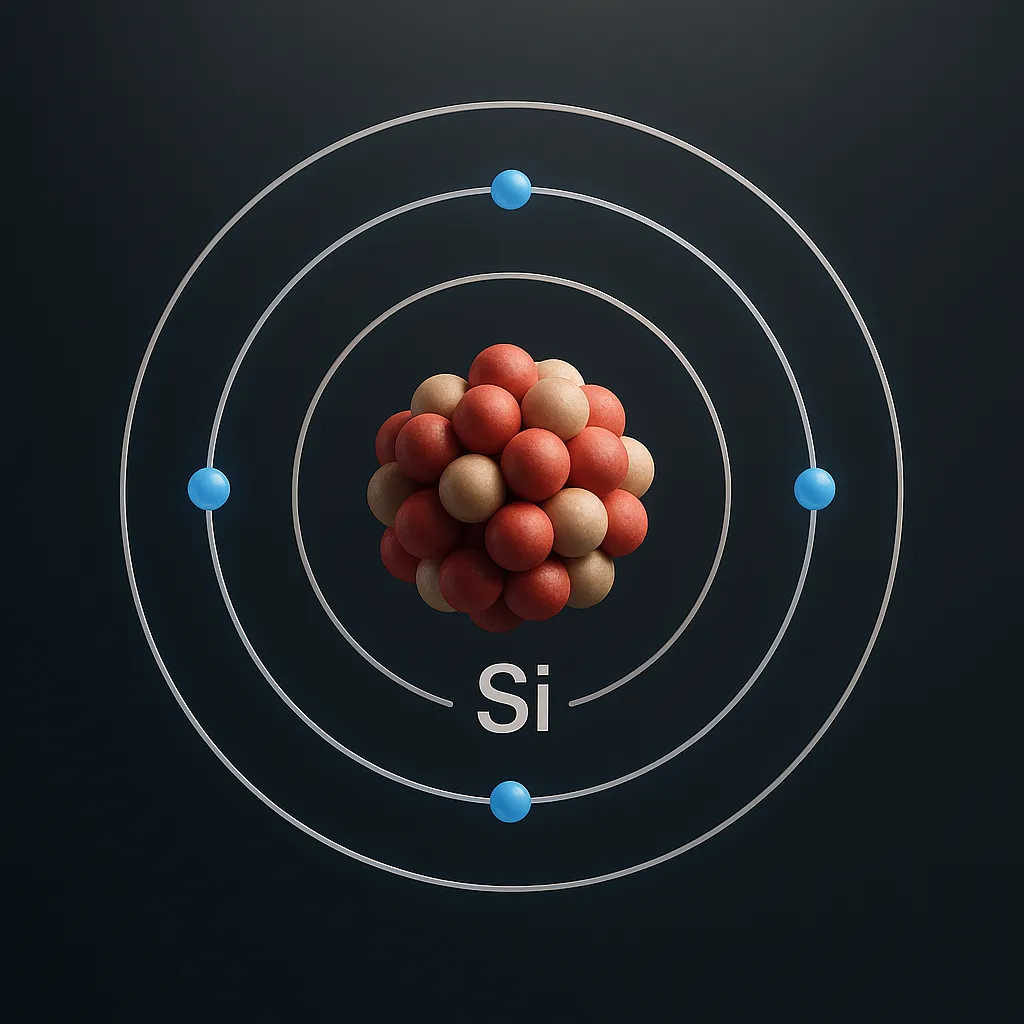
Silicon, a dark gray element with a metallic shine, is the backbone of modern technology. With the chemical symbol Si and atomic number 14, it’s the second most abundant element in Earth’s crust, making up 27.7% of it. This element powers the devices we use every day. From smartphones to solar panels, the applications of silicon are vast. As of May 2025, silicon’s role in electronics and renewable energy continues to grow.
This article explores silicon evolution, focusing on the applications of silicon. We’ll dive into its origins, properties, and its impact on our world today. Whether you’re curious about tech or sustainability, silicon’s story is one worth knowing.

The Discovery of Silicon
Silicon evolution began centuries ago, though its potential wasn’t realized until later. In 1823, Swedish chemist Jöns Jacob Berzelius first isolated silicon by heating potassium with silicon tetrafluoride. At first, silicon was a scientific curiosity. Its name comes from the Latin word “silex,” meaning flint, due to its presence in rocks.
However, it wasn’t until the 20th century that the applications of silicon became clear. In the 1940s, scientists discovered silicon’s potential as a semiconductor. This led to the development of transistors, paving the way for modern electronics. As a result, silicon became a cornerstone of the tech industry.
Silicon’s Journey to Earth
Silicon has cosmic origins, much like other elements. Billions of years ago, it formed in the hearts of massive stars through nuclear fusion. When these stars exploded as supernovae, they scattered silicon atoms across space. Then, about 4.6 billion years ago, our solar system formed from a cloud of gas and dust containing these atoms.
On Earth, silicon settled into the crust, bonding with oxygen to form silicate minerals like quartz. These minerals make up over 90% of Earth’s crust, according to geological studies. Over millions of years, natural processes concentrated silicon in deposits, such as sand. Consequently, the applications of silicon rely on these abundant natural resources, which are mined globally.

Properties of Silicon
The applications of silicon are tied to its unique properties. Silicon has an atomic mass of 28.085 and belongs to group 14 in the periodic table, also known as the carbon group. At room temperature, it’s a solid with a crystalline structure that gives it a metallic sheen. One of its key traits is its role as a semiconductor.
The properties of silicon allow it to conduct electricity under certain conditions, making it ideal for electronics. For example, silicon can be “doped” with elements like phosphorus to enhance its conductivity. In addition, silicon is highly resistant to heat and chemicals, which is why it’s used in harsh environments. These properties of silicon make it a vital material in technology and industry.
Silicon in Our World Today
The applications of silicon are at the heart of modern life. The most famous use of silicon is in electronics. In 2023, over 90% of microchips in devices like smartphones and laptops were made from silicon, per industry reports. For instance, the chips in your phone rely on silicon to process data at lightning speed.
Additionally, silicon plays a key role in solar energy. About 95% of solar panels use silicon-based cells to convert sunlight into electricity, according to 2024 data. This has made renewable energy more accessible. It also contributes to quantum computing advancements. Researchers are using silicon to create quantum bits, or “qubits,” which could lead to computers far more powerful than today’s systems.
In 2024, IBM reported progress in silicon-based quantum chips, hinting at a future where quantum computing transforms industries. Meanwhile, silicon is being used in lithium-silicon batteries. These batteries, still in development, promise up to 40% higher energy density than traditional lithium-ion batteries, potentially revolutionizing electric vehicles by 2030.
Beyond tech, silicon is used in glass and ceramics, like windows and bottles. In construction, silicon makes concrete and sealants more durable. However, mining silicon has challenges. Extracting it requires energy-intensive processes that can impact the environment. On the other hand, silicon and the environment benefit from its use in green tech, like solar panels. These applications of silicon show its incredible versatility and future potential.

Leave a Reply