Introduction
Bromine, element 35 on the periodic table, is a feisty liquid with a reddish-brown glow and a sharp, nose-stinging smell. It’s one of just two elements that stay liquid at room temperature, giving it a quirky spot in the chemical lineup. Bromine element properties place it in the halogen group, where it’s a bit less wild than chlorine but bolder than iodine. From sanitizing pools to fireproofing gadgets, it’s a crafty chemical with big uses. This article dives into what Bromine is, how it’s used, how we get it, and what scientists are figuring out about it.
You won’t catch Bromine chilling solo in nature—it’s too reactive, always teaming up with other elements. It hides in salty seawater or underground brines, popping up in industry and even tiny roles in living things. Bromine element properties make it both a helper and a handful, demanding careful handling. Let’s unpack this lively element’s story. The sections below explore its traits, uses, and some neat science behind it.

What Brom Old Bromine Is All About
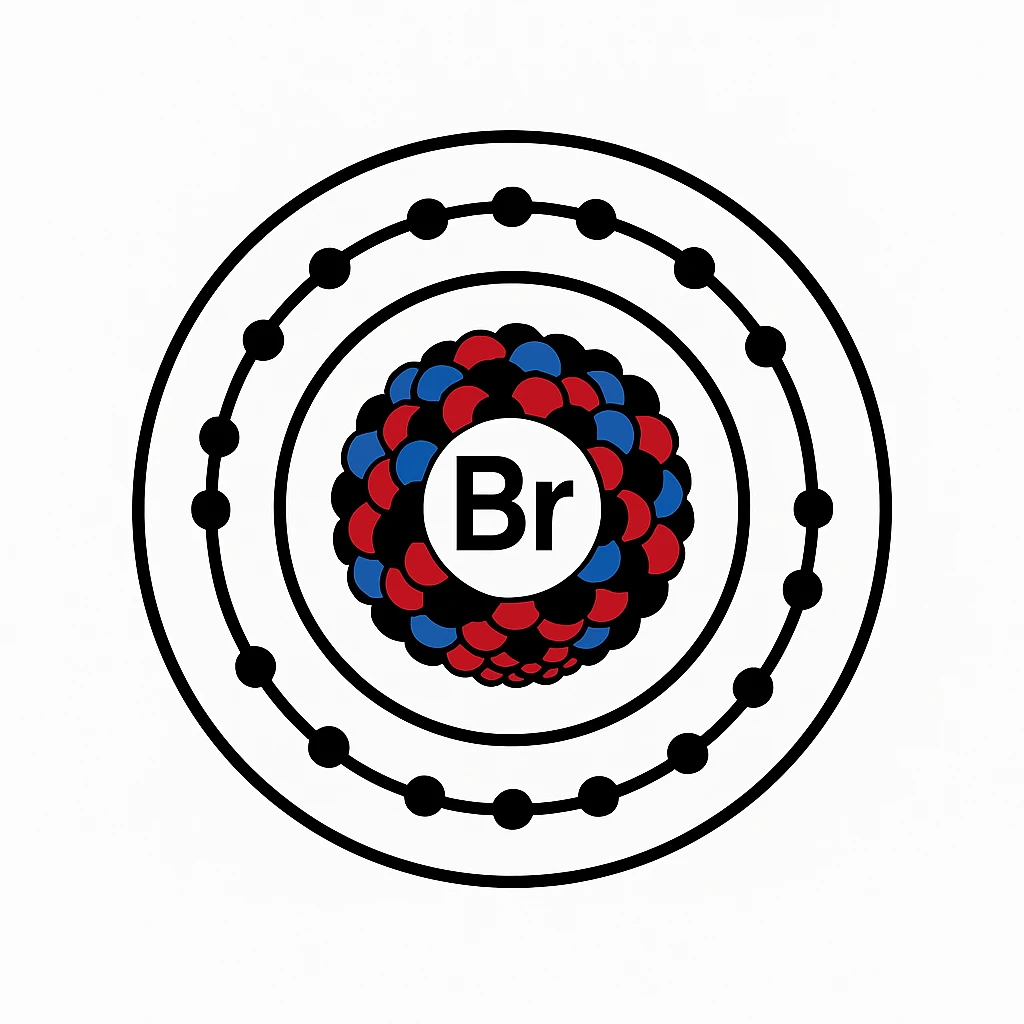
Bromine sits in the halogen group on the periodic table with 35 protons and an atomic weight of about 79.9. As a nonmetal, it’s a liquid at room temperature, sporting a deep red-brown color and a pungent smell that can irritate skin or lungs if mishandled. Bromine element properties drive its high chemical reactivity, meaning it eagerly snags electrons to form compounds, never existing alone in nature. It’s typically found in salty minerals or ocean water, making it a chemical that loves company. Its reactivity requires careful storage in glass or lead-lined containers to avoid corrosion or burns.
The halogen group links Bromine with fluorine, chlorine, and iodine, and it’s a middle child in terms of chemical reactivity—less intense than chlorine, more so than iodine. Its seven outer electrons fuel its bonding urge, making it a strong oxidizer for chemical reactions. Bromine’s liquid state is rare, shared only with mercury at room temperature, and its vapor is just as harsh as the liquid. Two stable isotopes, Br-79 and Br-81, split evenly in nature, helping scientists track it in compounds.
This reactivity makes Bromine a double-edged sword: incredibly useful but tricky to handle. Its fumes can corrode metals, and accidental exposure can sting, so safety gear like gloves and masks is a must. Bromine element properties give it a unique role in the periodic table, balancing reactivity and utility. It’s a key player in chemistry, bridging its halogen siblings with distinct flair.
Why Bromine Matters
Bromine’s a heavy hitter because it forms bromine compounds used across industries. It’s in flame retardants, slowing fires in furniture and electronics, and in pool sanitizers, knocking out bacteria like a quieter chlorine. Bromine element properties, especially its chemical reactivity, make it perfect for these roles. It’s also in some medicines, dyes, and even old-school photography, where silver bromide catches light for images. Its versatility keeps it in demand.
In nature, Bromine lurks in seawater and salty underground pools, but it’s scarce in Earth’s crust. Its ability to bond with most elements—except those aloof noble gases—makes it a chemical champ. However, its environmental impact is a concern; some bromine compounds, like certain pesticides, harm the ozone layer, which shields Earth from UV rays. Global rules now limit these uses, pushing for safer options. Bromine compounds in soil or water can also linger, affecting plants and animals.
The challenge is balancing Bromine’s perks with its risks. Its reactivity demands careful use to avoid environmental trouble, like pollution from improper disposal. Bromine element properties mean it’s a helper when controlled, but a troublemaker if let loose. Its role in industry and nature makes it a chemical worth watching closely.

How Bromine Gets to Us
Bromine extraction pulls this element from salty sources like seawater or underground brines. Chlorine gas is used to bump Bromine out of bromide salts, creating a weak solution that air or chemicals concentrate into pure, reddish liquid. Bromine element properties, like its solubility, make this process effective, especially in areas with super-salty water. The liquid is then stored in glass or lead-lined tanks to prevent corrosion or burns, as its vapors can eat through metal. Safety is critical since mishandling can cause skin or lung irritation.
The process is slick but has environmental catches. Leftover chemicals from extraction can pollute water or soil if not managed well, adding to Bromine’s environmental impact. Some methods recycle Bromine from industrial waste, which is kinder to the planet. Strict controls keep leaks or spills in check, given Bromine’s reactive nature. Producers refine these techniques to boost efficiency and cut harm.
Bromine extraction needs precision to balance output and safety. The reactivity that makes Bromine useful also makes it a handful, requiring specialized gear and know-how. Bromine element properties drive both the process and its challenges, making extraction a careful dance. It’s all about getting this potent element without leaving a mess.

What Science Is Learning About Bromine
Scientists digging into Bromine element properties are uncovering its hidden sides, especially in biology and environmental fixes. Bromide ions, a Bromine form, might help animals strengthen tissues like collagen in skin or bones, and tiny amounts could aid white blood cells in fighting parasites. These ideas are early but suggest Bromine has a sneaky role in life. Researchers are testing how these ions work without tipping into toxicity, given Bromine’s punch.
Environmental studies focus on Bromine’s harm to the ozone layer, as some bromine compounds are worse than others at thinning this protective shield. Scientists are pushing for safer flame retardants and testing traps for Bromine vapors to curb air pollution. These efforts aim to keep Bromine’s benefits while dodging its environmental impact. New tech could make industries cleaner, reducing Bromine’s footprint in water and soil.
The wildest research asks if Bromine’s ability to slow reactions could spark medical breakthroughs, like preserving tissues or calming overactive body systems. These studies are just starting, but Bromine element properties hint at uses beyond sanitizers and plastics. From biology to green tech, the science is alive with ideas, showing Bromine’s got more tricks up its sleeve.

Leave a Reply