Introduction
Ever thought about calcium element uses? It’s pretty amazing! Calcium, element 20, is a silvery metal. It strengthens bones, teeth, and even powers industries. As of May 2025, calcium remains a star in science and health. So, this article dives into calcium element uses. We’ll explore its spot in the periodic table, properties, discovery history, and applications. Ready to learn more? Let’s get started!
Calcium’s everywhere, from your body to nature! About 3.5% of Earth’s crust is calcium. Why’s it so important? Stick around to see how this metal makes life better.
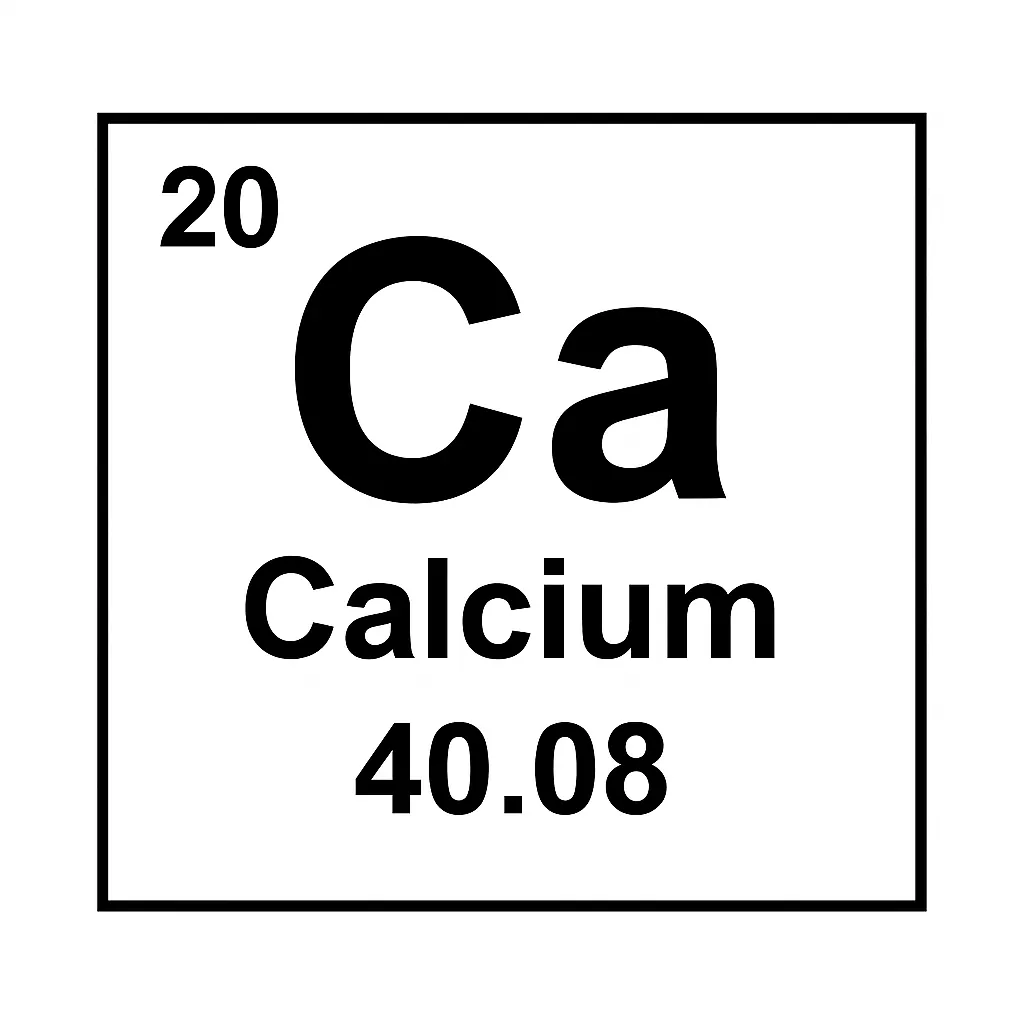
Where Does Calcium Fit in the Periodic Table?
Calcium in periodic table sits in Group 2, with alkaline earth metals like magnesium. That means it has 20 protons and 20 electrons. Its electron setup is [Ar]4s², making it quite reactive. It loves losing its two outer electrons to form compounds. Besides, it’s in Period 4, right below magnesium, sharing similar traits.
It’s super abundant—about 3.5% of Earth’s crust by weight. Calcium in periodic table mostly appears as calcium-40, which is stable. Also, calcium-48 is a rare radioactive isotope used in research. This reactivity makes it valuable. Let’s check its properties next to understand why it’s so special.

What Are Calcium’s Properties Like?
Calcium properties are honestly pretty cool. It’s a soft, silvery metal that’s easy to cut. Reacting with water, it forms calcium hydroxide and hydrogen gas. That reaction isn’t explosive like some metals, but it’s steady. It melts at 842°C and boils at 1484°C, so it’s solid at room temp. Plus, its density is 1.55 g/cm³, making it fairly light.
These properties stem from its reactivity, thanks to those two outer electrons. Oxidizing in air, it forms a white oxide layer. That’s why it’s often stored away from moisture. Calcium properties also include conducting electricity, which is useful in some applications. Hence, its traits make it versatile. Let’s see how this metal was discovered next!

How Was Calcium Discovered?
Calcium discovery history goes back to 1808. A British chemist, Humphry Davy, made it happen. He used electrolysis on lime—calcium oxide mixed with mercury oxide. Passing an electric current through it, he isolated pure calcium. That was a big moment, marking the first time anyone saw this metal!
Before Davy, people used lime in construction, like in ancient Roman cement. Yet, no one knew it contained a new element. So, calcium discovery history confirmed it’s an alkaline earth metal. Davy’s work built on his earlier discoveries, like sodium and potassium. Consequently, he became a science legend. How cool is that? Now, let’s explore what calcium does for us today.

What Are Calcium Element Uses and Safety Tips?
Calcium element uses are all around us, and they’re super important. First, it’s a key player in your body. It builds strong bones and teeth—99% of your body’s calcium is there! It also helps muscles contract and blood clot. You get it from foods like milk, yogurt, and spinach—adults need about 1,000 mg daily. Doctors often prescribe calcium supplements for bone health.
Industry loves this metal too. Calcium element uses include making cement, steel, and aluminum alloys. It’s also in batteries, like calcium-lead ones for cars, which are gaining popularity in 2025. Farmers use calcium in lime to reduce soil acidity, helping crops grow better. Also, it’s in antacids to soothe stomach acid. However, safety matters—it reacts with water, so handle it dry. It can irritate skin or eyes, so wear gloves. Store it away from moisture to avoid reactions. Thus, calcium element uses are awesome, but careful handling keeps things safe.
Leave a Reply